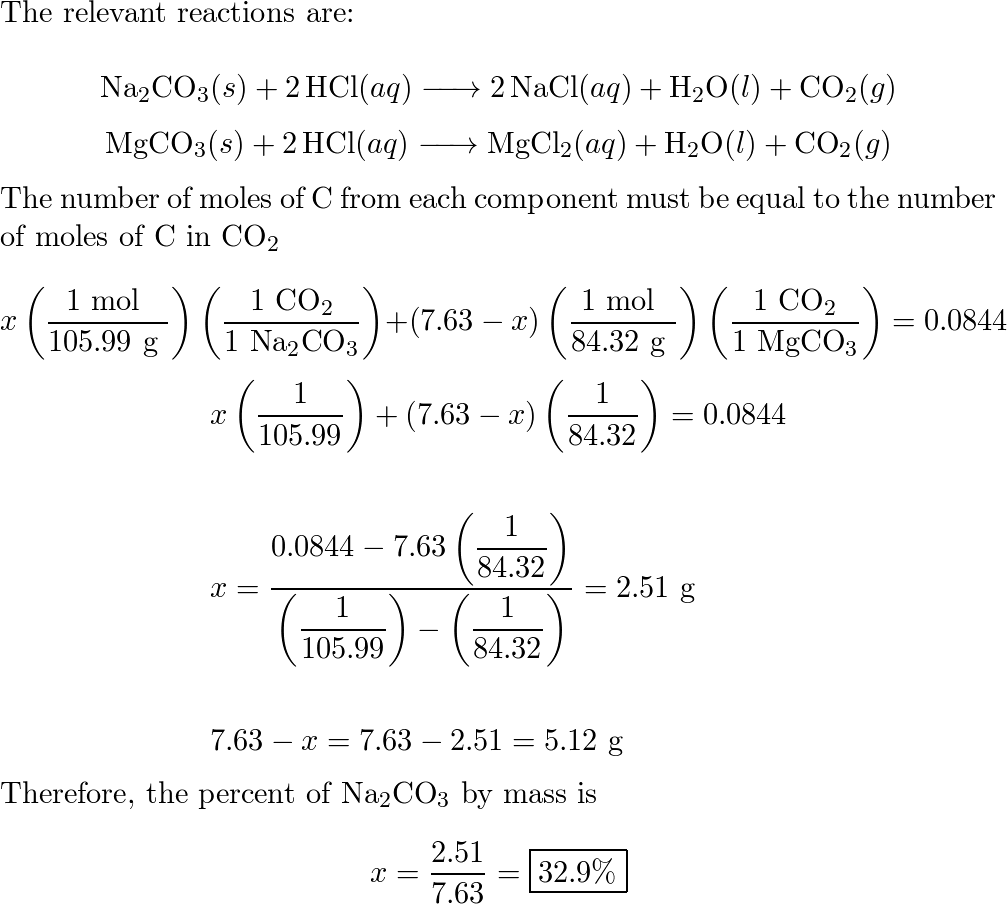⚗️This chemical equation represents a chemical reaction. Na2CO3 + 2HCl - 2NaCl + CO2 + H20 Which - Brainly.com
40. A 2g sample containing Na2CO3 and NaHCO3 loses 0.248g when heated to 300^° c,the temperature at which NaHCO3 decomposes to Na2CO3,CO2 AND H2O.what is the precentage of Na2CO3 in the given

CO2 capture by aqueous Na2CO3 integrated with high-quality CaCO3 formation and pure CO2 release at room conditions - ScienceDirect
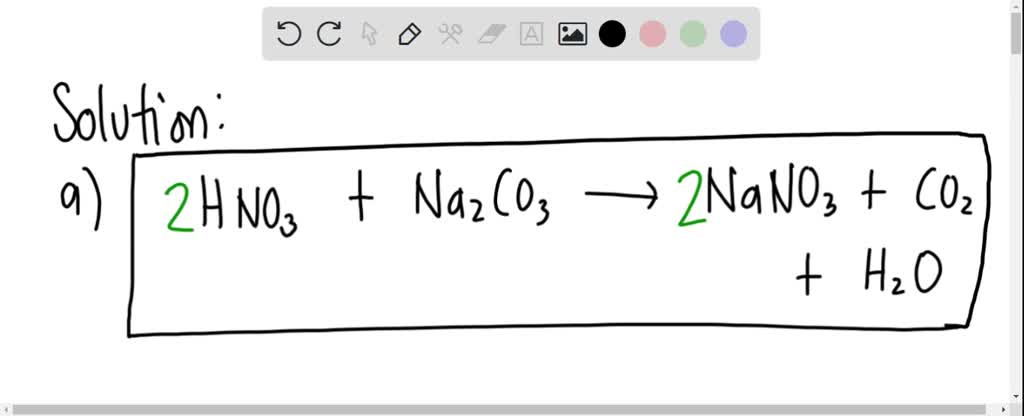
SOLVED: Balance the equation HNO3+Na2CO3—->NaNO3+CO2+H2O If you have 750.0 g of HNO3 and excess Na2CO3, how many grams of carbon dioxide will be produced?
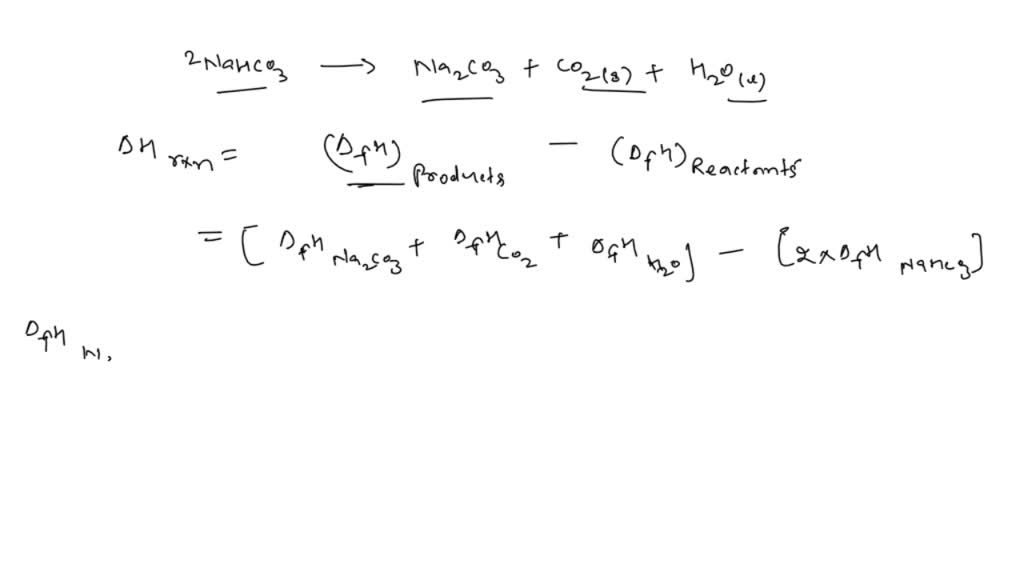
SOLVED: use Hess's law to calculate the enthalpy of reaction for reaction 6: 2 NaHCO3 (s) → Na2CO3 (s) + CO2 (g) + H2O (l). Show your workings

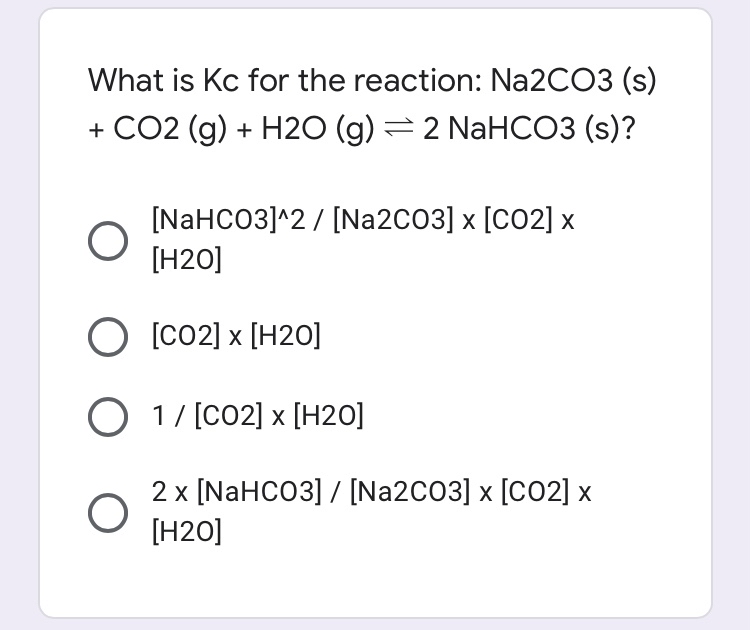


![ANSWERED] Which equation represents a non-oxidatio... - Inorganic Chemistry ANSWERED] Which equation represents a non-oxidatio... - Inorganic Chemistry](https://media.kunduz.com/media/sug-question/raw/62112503-1657345217.6842966.jpeg)