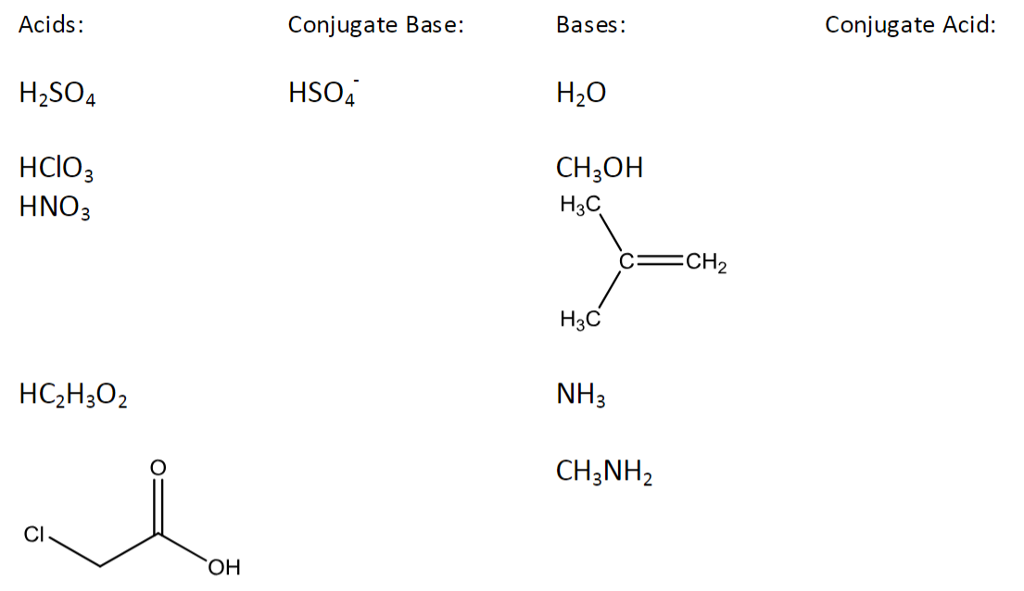
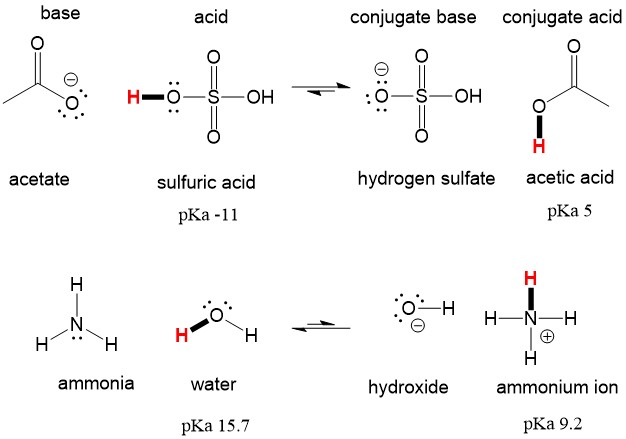
Write a balanced equation for the dissociation of the following in water and identify the conjugate acid - base pairs. (i) NH4^(+) (ii) H2SO4 (iii) CH3COOH
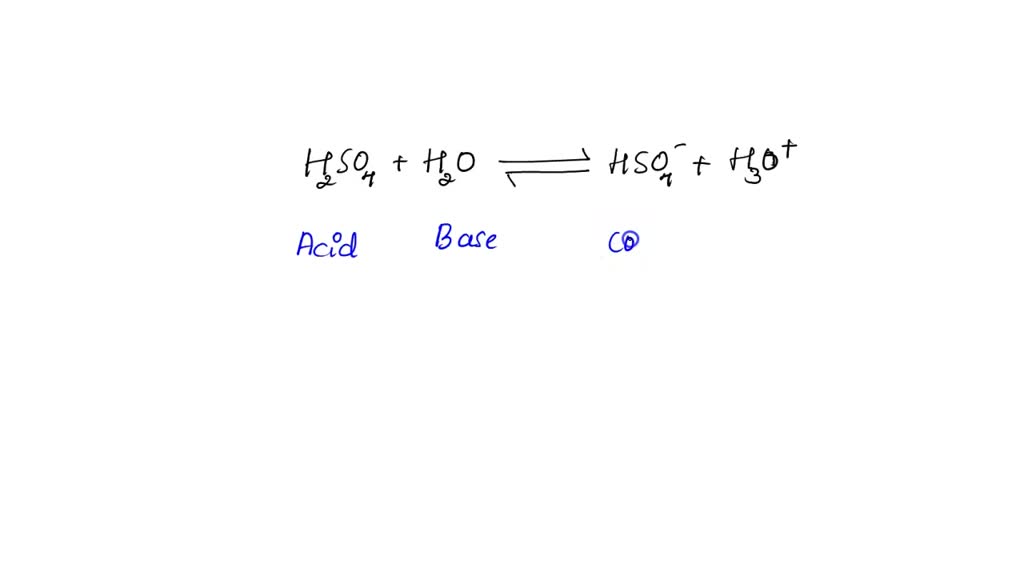
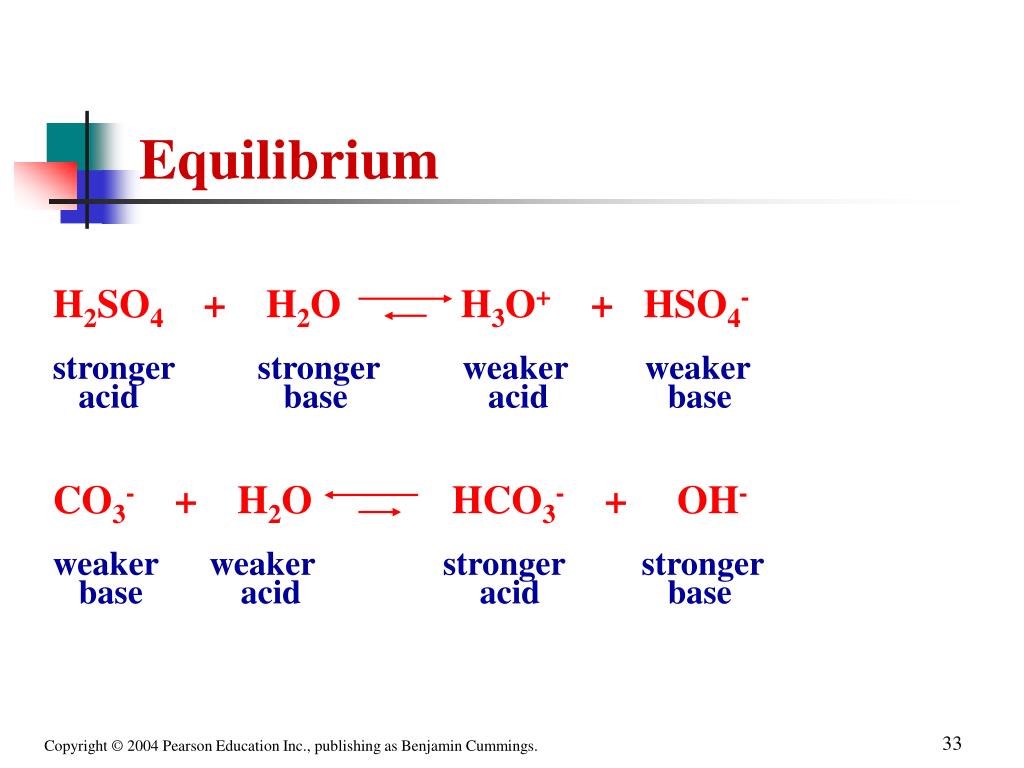
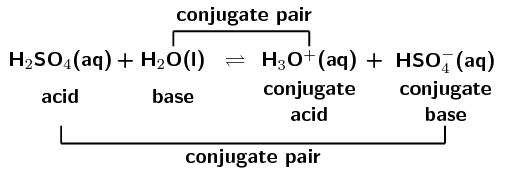
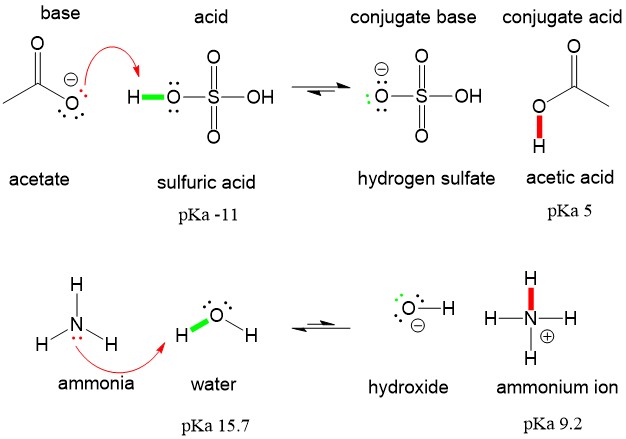
SOLVED: In the following equation, identify the Bronsted Lowry conjugate base H2SO4 + H2O <-> HSO4 - + H3O+ Group of answer choices H2SO4 HSO4- H2O H3O+
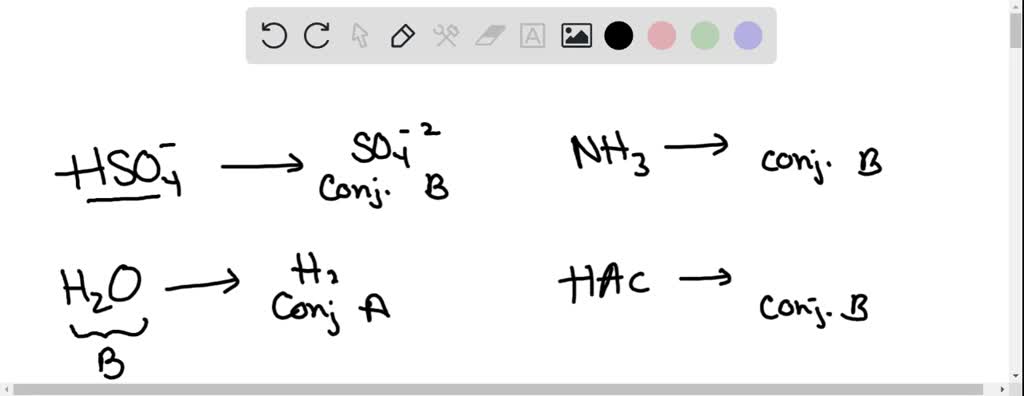
SOLVED: Which of the following is a CORRECTLY matched pair?Required to answer. Single choice. conjugate base of HSO4- is H2SO4 conjugate acid of H2O is OH- conjugate base of NH3 is NH4+

Identify the acid-conjugate base pair in this balanced equation: H2SO4 + 2NaOH → 2H2O + - Brainly.com