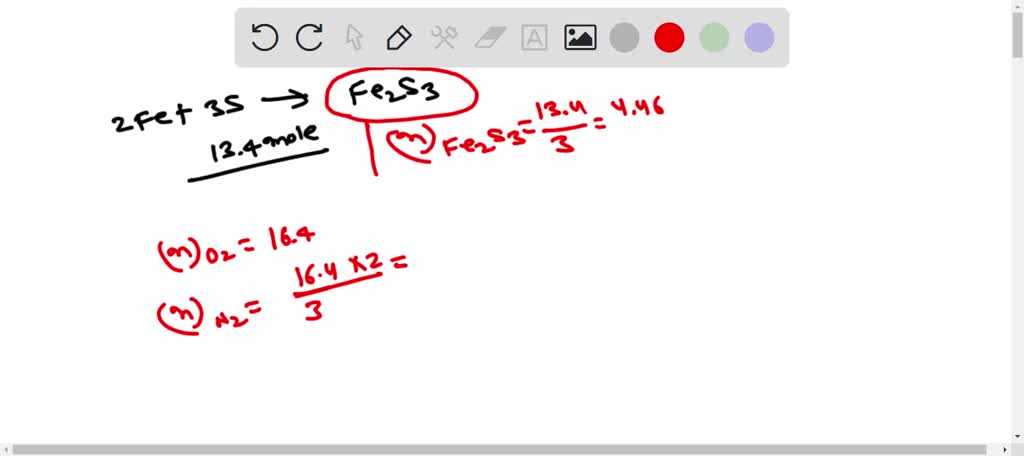
SOLVED: 1. How many moles of Fe2S3 can form from 13.4 moles of S? 2Fe +3S –> FE2S3 A. 40.2 Moles B.4.47moles C.0.224 moles D.13.4 moles 2. How many grams of N2
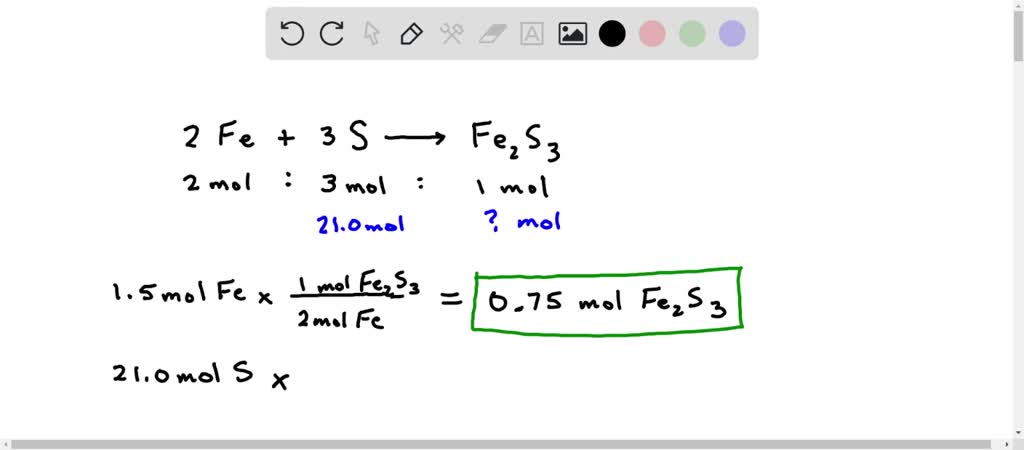
SOLVED: 1A. Consider the balanced chemical equation that follows: 2Fe(s) + 3S(s) → Fe2S3(s) This equation can be read as 2 moles of Fe + 3 moles of S → 1 mole

Equivalent weight of Fe2S3 in the reaction is :(M = molecular weight of Fe2S3 ) Fe3S3 + O2⟶FeSO4 + SO2
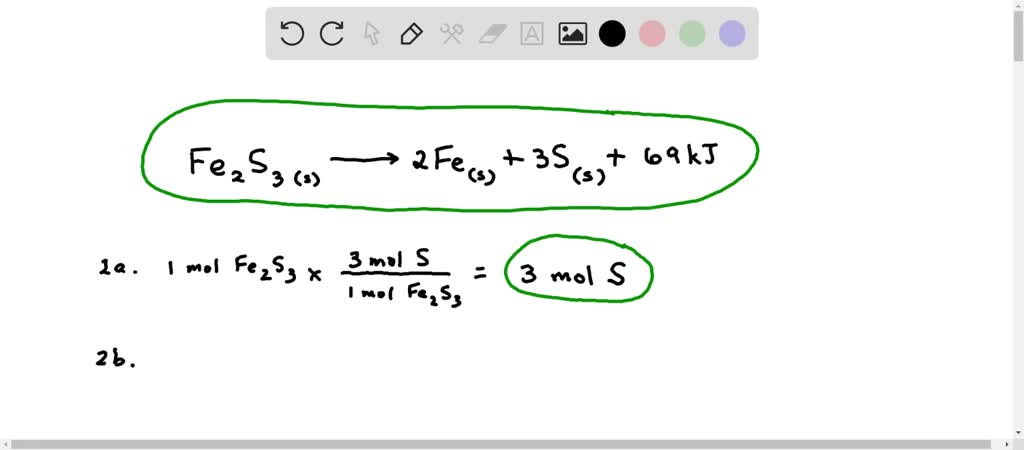
SOLVED: Balance the equation Fe2S3(s) → Fe(s) + S(s) + 69kJ 1b) 2A. How many moles of sulfur are in one mole of Fe2S3(s)? 2b. How many moles of Sulfur is in

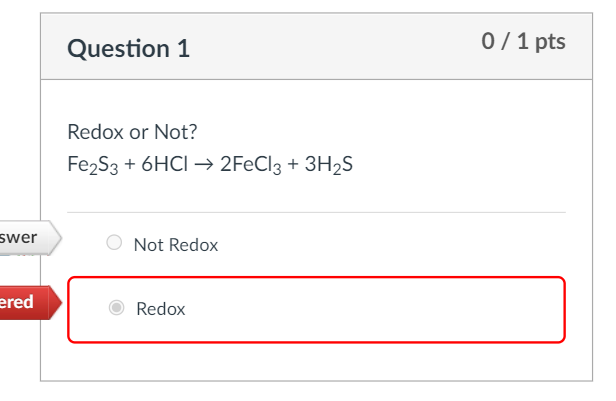
If the reaction occurs between Fe2S3(s) and H+ (aq) and the equation is properly balanced with the - Brainly.com