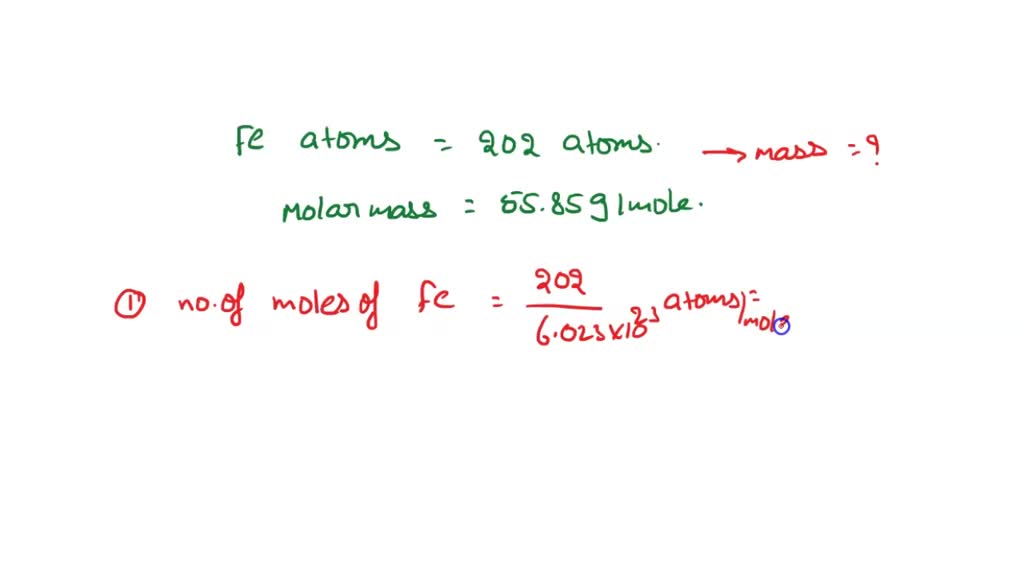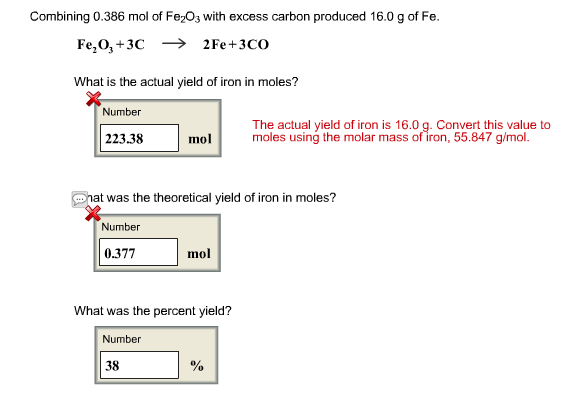
Bling Bling: if I were to give each of you one atom of gold for every second that has elapsed since the Dinosaur's went extinct 65 million years ago, how. - ppt download

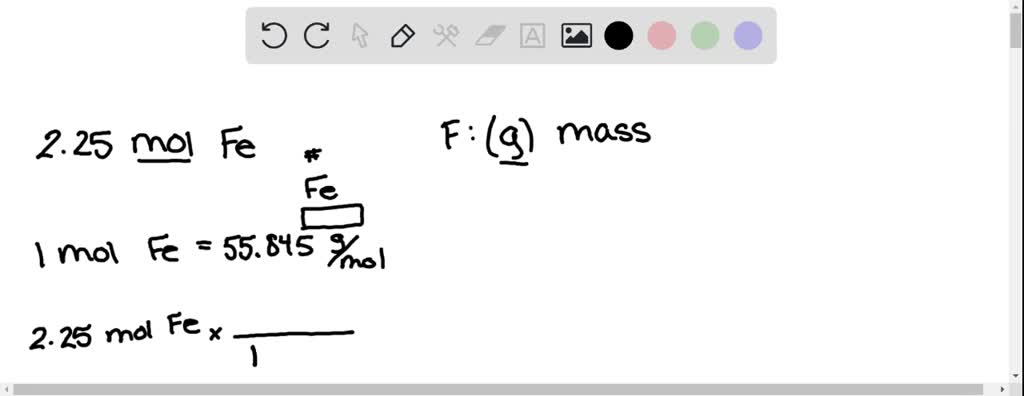
Jericho Rayel Timbol on Twitter: "Now let us to moles to mass ratio. Consider the reaction A → B Mass A can be converted to Mass B given that the molar masses

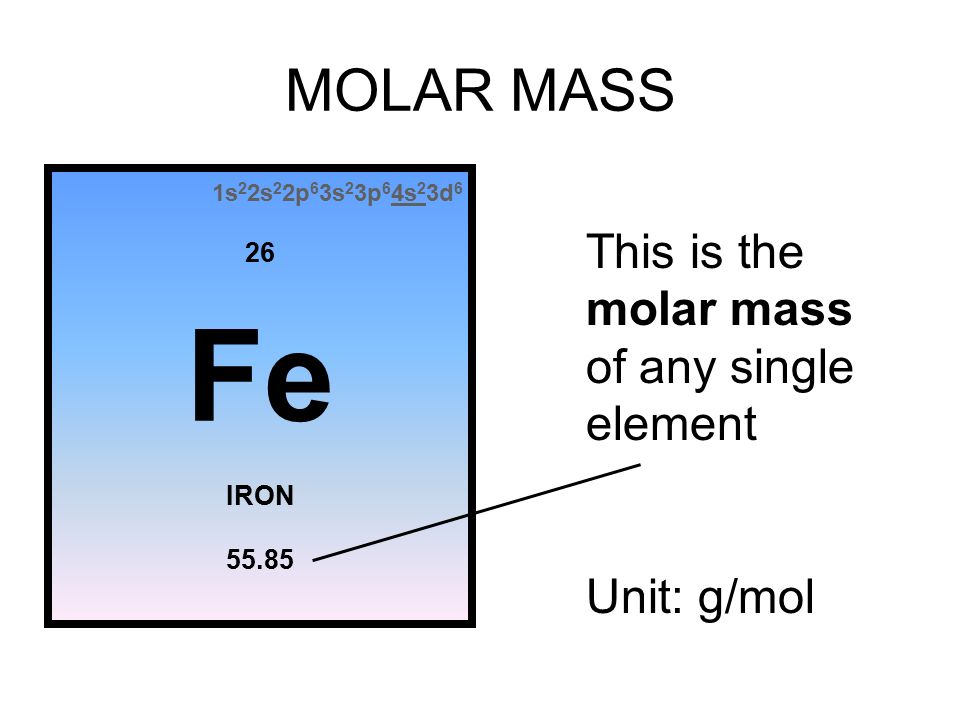
Once you know the number of particles in a mole (Avogadro's number = 6.02 x ) and you can find the molar mass of a substance using the periodic table, - ppt download