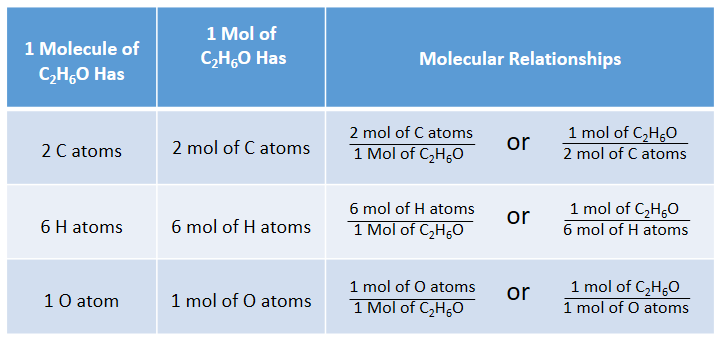
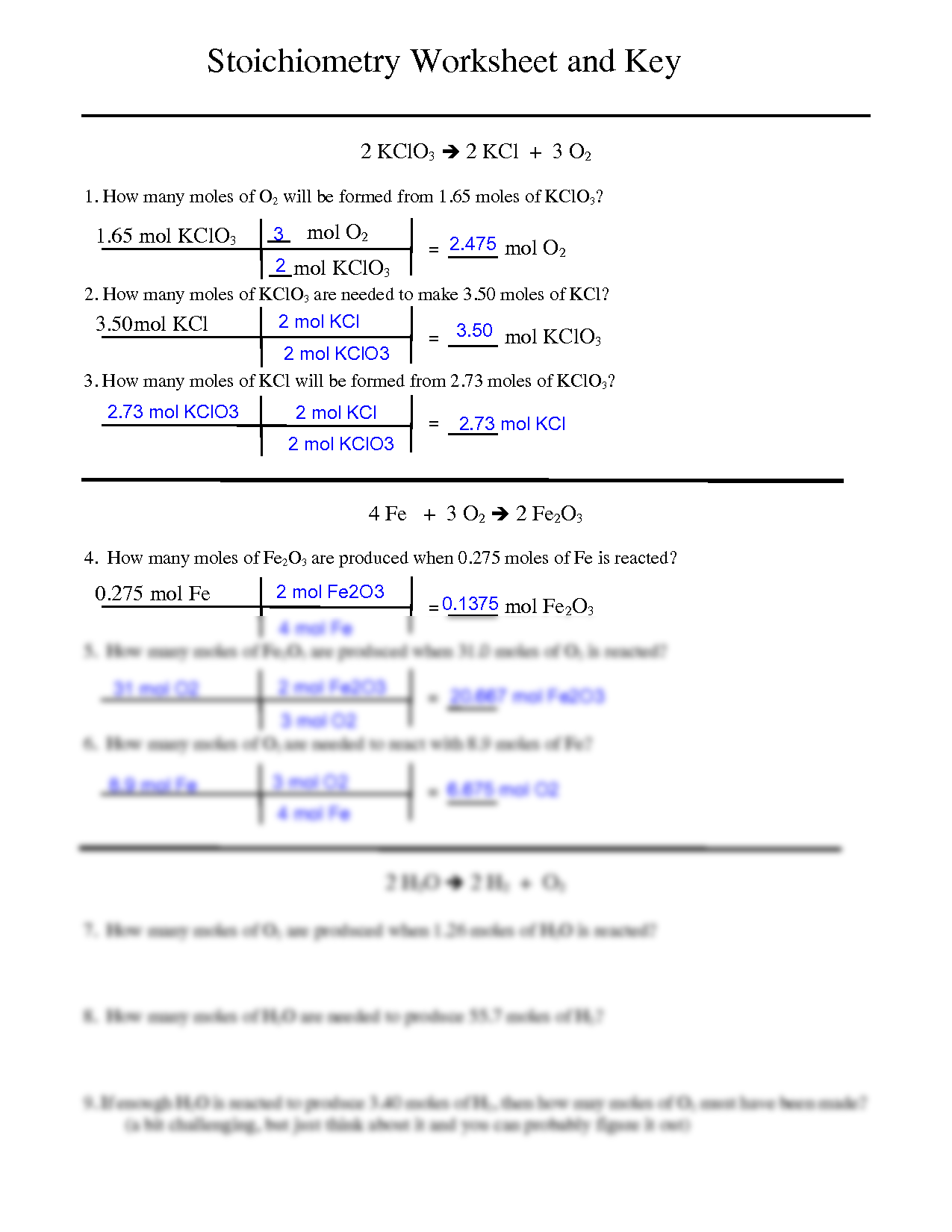
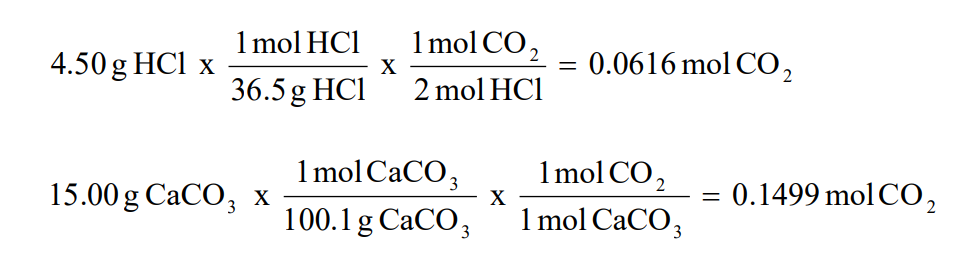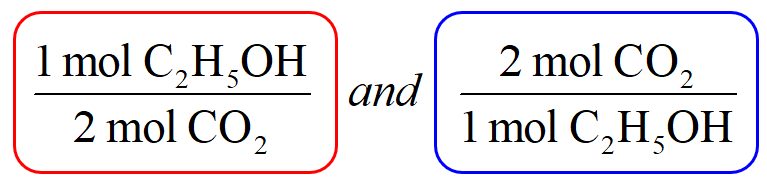Question Video: Identifying a Precipitating Agent for the Gravimetric Analysis of Chloride Ions | Nagwa

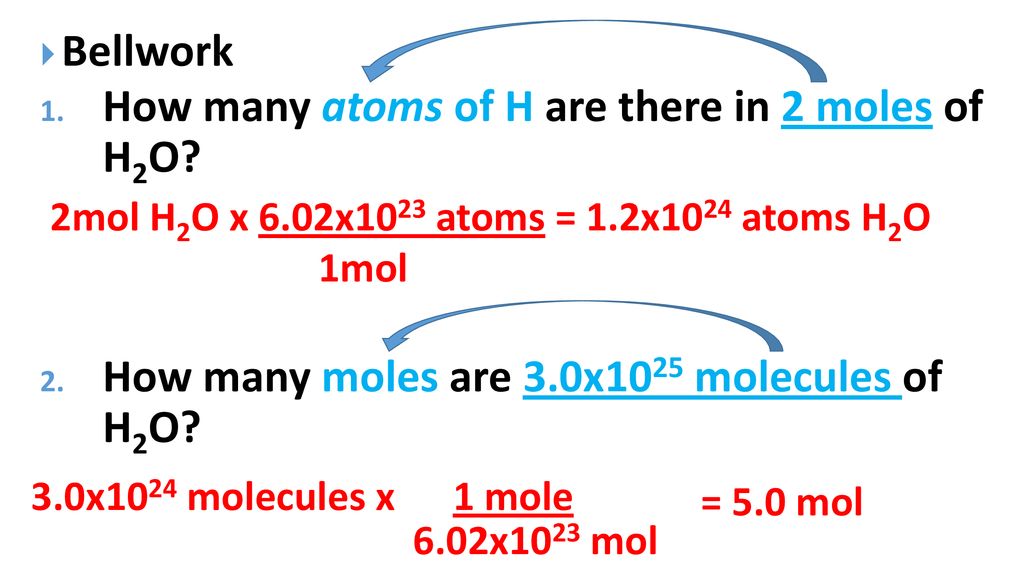
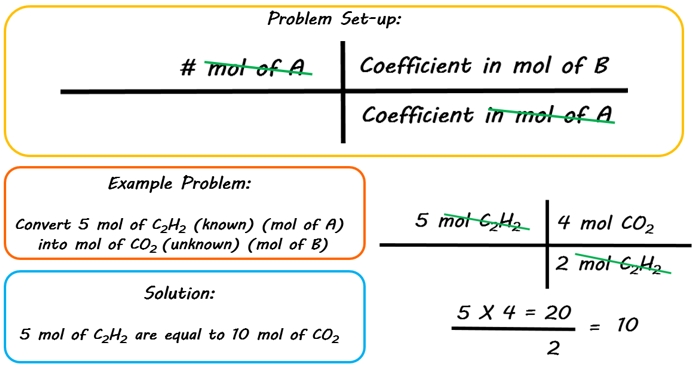
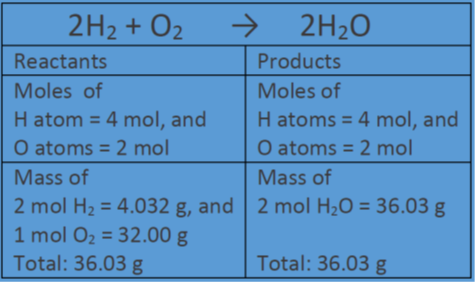
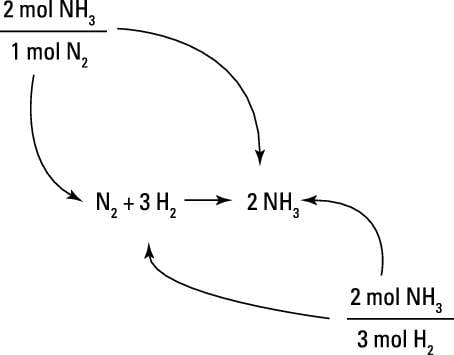
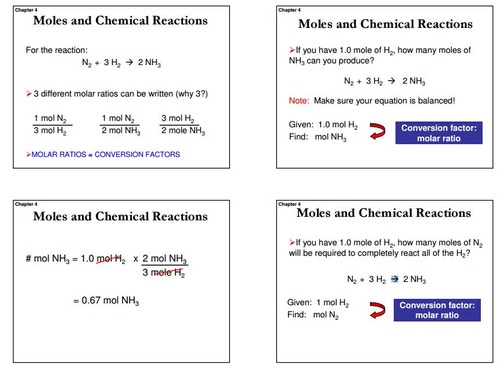
Mol ratio: coefficients of a balanced equation 2 H 2 + O 2 → 2 H 2 O 2 mol H 2 for every 1 mol O 2 In chemical calculations, mol ratios convert moles of. - ppt download

Question Video: Calculating the Percentage by Mass of Water in Alum Given Its Chemical Formula | Nagwa

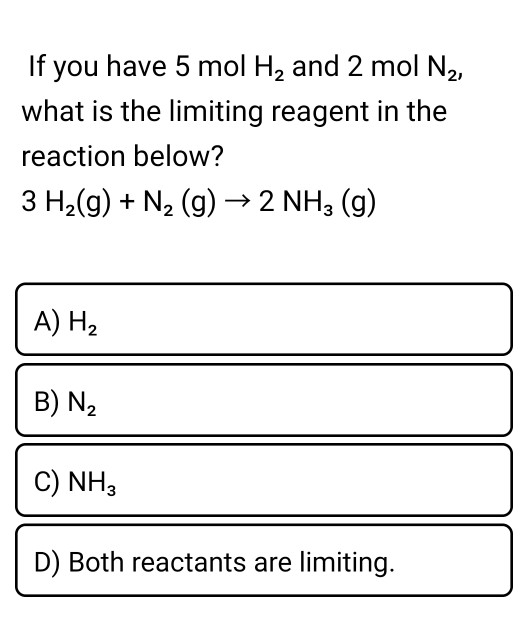
1 mole of N2 and 2 moles of H2 are allowed to react in a 1 dm^3 vessel. At equilibrium 0.8 mole of NH3 is formed. The concentration of H2 in the vessel is